WEBINAR
Effective Data Integrity Program - A structured approach
On 14th October 2020 Wednesday 8:30 PM IST
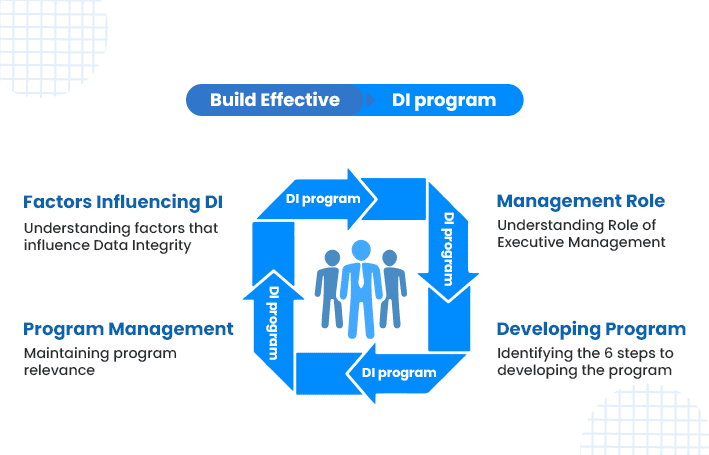
The webinar is designed for establishing a Data Integrity (DI) program.
With a holistic view of a company’s operation, the webinar will highlight DI’s 5-p model that global companies are effectively using to minimize exposure to data integrity audit failures..
After a compendium of DI fundamentals, the webinar will quickly transition to business process considerations and interactions between people, process and equipment coupled with company culture, propensity for humans to make mistakes along with reasons, etc.
Speaker Introduction

Mr. Chinmoy Roy
Subject Matter Expert in CSV and Data Integrity
Chinmoy Roy is a seasoned life science professional. He is a Subject Matter Expert in CSV and Data Integrity.
However, his skill set that crosses over in multiple areas from engineering to manufacturing IT to laboratory systems, validation, and quality processes.
He has over 40 years of field experience and uses it to help companies worldwide. While employed at Genentech, he led and directed a team of 50+ people in the design, installation and obtaining “fit for use” certification for the world’s largest paperless biopharmaceutical manufacturing facility.
In his current consulting role, he conducts Data Integrity audits, some of which he teams up with former FDA auditors. He has assisted companies is establishing Data Integrity programs, training employees in Data Integrity Best Practices. In 2019, his lucid presentation style was awarded the “Best Speaker of the Year” by IVT, USA
Discussion panel

Timur Kabadayi,
Managing Director, CONVALgroup

Banu Refik,
Senior Consultant Quality, CONVALgroup

Arjun Guha,
Director Operations, CONVALgroup
What will you take away from the session?
- Pharma 4.0 and DI by Design
- Why DI issues occur
- What is the 5P model for DI
- Recipe for DI program success


